July 2025: New Study Published!
Our latest population-based study confirms that genotype-specific HPV mRNA testing with PreTect HPV-Proofer 7 offers superior specificity and risk stratification compared to cytology in HPV DNA-positive women.
✅ 47% relative improvement in positive predictive value (PPV)
• 28.1% with mRNA vs. 19.1% with cytology
✅ Substantially higher specificity
• 72.3% with mRNA vs. 53.0% with cytology
✅ 31% reduction in colposcopies per CIN2+ detected
• 3.6 with mRNA vs. 5.2 with cytology
✅ Comparable and high negative predictive value (NPV)
• 94.6% with mRNA vs. 93.0% with cytology
—All achieved without compromising diagnostic sensitivity
By detecting transcriptionally active HPV infections, the mRNA test enables more precise clinical decisions. This allows for fewer unnecessary procedures and a more targeted, cost-effective approach to cervical cancer prevention.
📖 Read the full study in Pathogens:
https://doi.org/10.3390/pathogens14080749
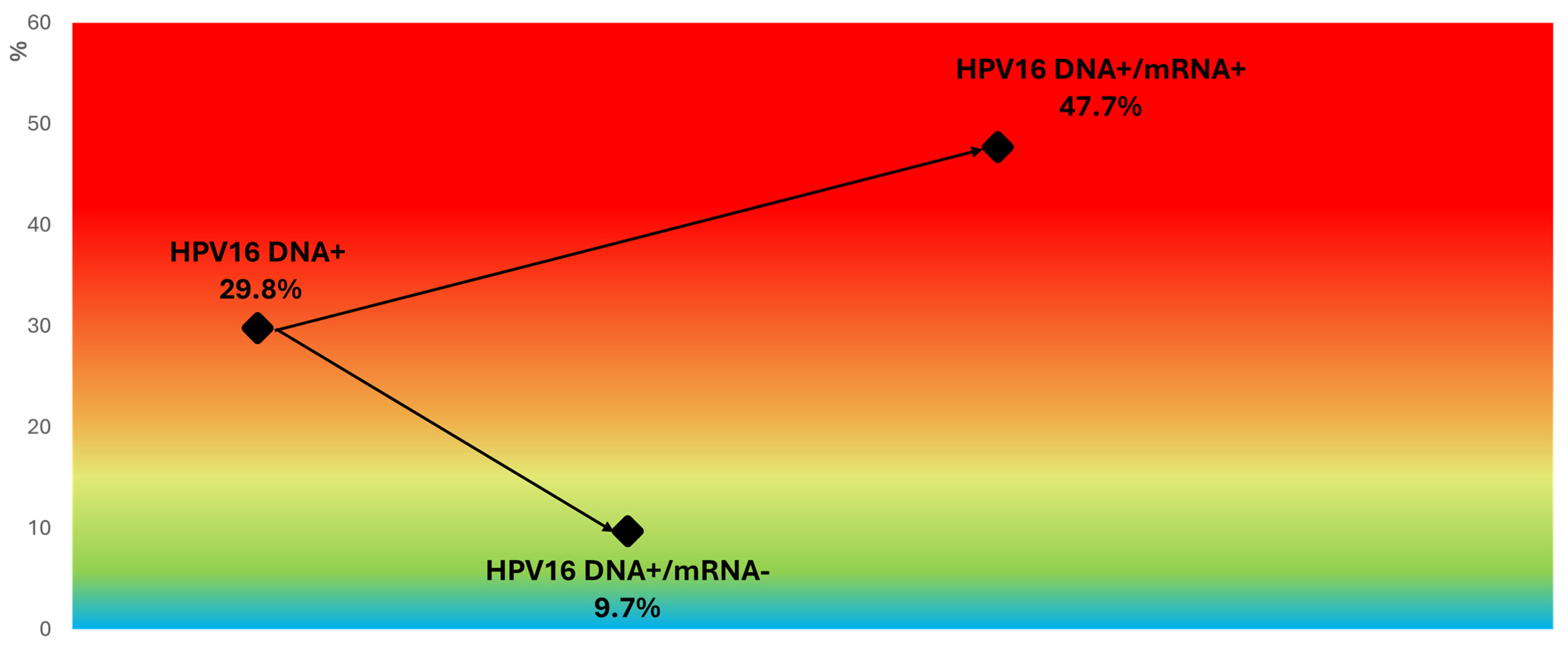
Effective Stratification of CIN2+ Risk - even in HPV16-Positive Women:
Figure 5 from our recent Pathogens publication highlights how genotype-specific mRNA testing refines clinical decision-making, within the HPV16 DNA-positive group.
Women who are positive for both HPV16 DNA and mRNA16 represent a high-risk subgroup warranting immediate referral to colposcopy and biopsy.
In contrast, the dramatic drop in risk observed among HPV16 DNA-positive but mRNA-negative women supports a more conservative, individualized management approach—particularly valuable for younger women, where regression is common and active surveillance is often preferable.
09 August 2024
New Study Highlights the Robustness of HPV mRNA Testing in Cervical Screening
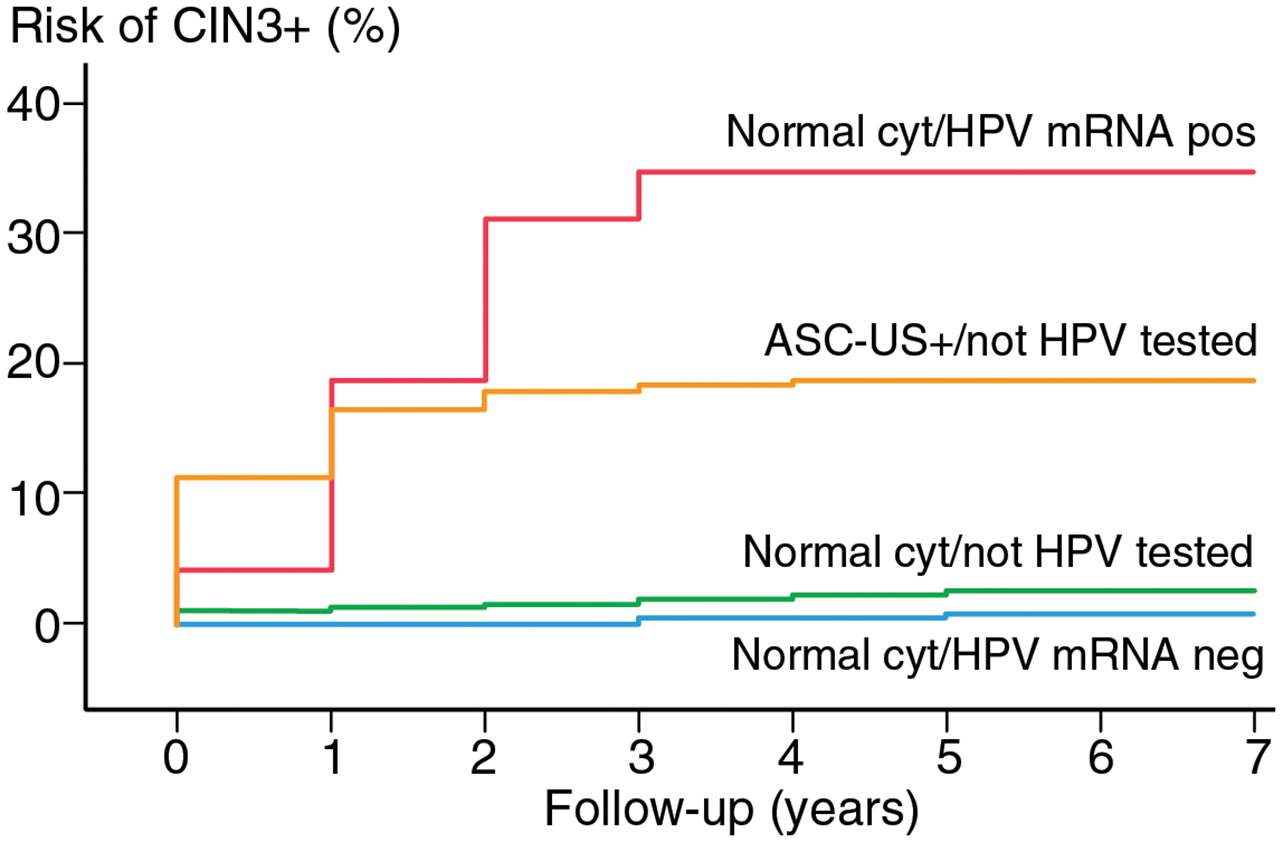
We are excited to share the findings of a newly published study that underscores the effectiveness of the PreTect SEE (HPV mRNA E6/E7 for types 16, 18, and 45) in real-world cervical screening. Conducted at the University Hospital of North Norway (UNN) between 2016 and 2019, this large-scale study analyzed 98,648 cervical samples, providing robust evidence of the test's utility.
Among the 61,635 women with normal cytology, 752 (1.2%) tested positive for HPV mRNA. Notably, while 70.7% of these women retained normal cytology upon re-evaluation, biopsies revealed a significantly higher risk for high-grade lesions (CIN2+) in 34% of cases and severe lesions (CIN3+) in 14%, including five cases of cervical cancer.
These results highlight the limitations of cytology alone and demonstrate the importance of HPV mRNA testing in identifying women at risk for serious cervical lesions, even when cytology appears normal. The study further reinforces the enhanced specificity of HPV mRNA testing compared to DNA testing, particularly in younger women, where transient infections often lead to false positives with DNA-based tests.
This research not only validates the integration of HPV mRNA testing within existing screening protocols but also suggests its broader applicability to improve cervical cancer screening programs globally.
[Read the full article here: https://www.mdpi.com/2673-4184/4/3/22]
08 July 2024: Excited to share our latest publication in MDPI Cancers on extended HPV mRNA genotyping by Proofer7 and self-sampling!
This breakthrough not only increases access to screening but also provides actionable results for clinicians. By reducing the number of colposcopies while detecting the same cases of disease, we're paving the way for more efficient and accessible healthcare.
Read more about our findings and innovations here: https://www.mdpi.com/2072-6694/16/13/2485
17 November 2023:
We are excited to share the HPV mRNA results from the DiaVACCS trial, which evaluated the potential preventive value of a "test-and-treat" strategy to impact cervical cancer prevention in a South African population, characterized by a high prevalence of women living with HIV (WLWH).
Despite being preventable, cervical cancer remains a lead killer among women in low-middle-income countries. The reasons are clear. Global inequity is reflected in limited health resources, such as limited access to HPV vaccination, screening, and treatment, which are the key pillars for the elimination of cervical cancer. The high burden of HPV-coinfections in women living with HIV/AIDS challenges the use of sensitive HPV testing, leading to massive over-treatment.
As advised by the WHO, a “test-and-treat” strategy could be optimal. In this study, 710 under-screened South African women, about half of them living with HIV, were tested for mRNA expression restricted to the predominant HPV types of cervical cancer. The effectiveness of a test-and-treat strategy to detect and manage severe cervical abnormalities (CIN3+) by increasing the number of HPV types included in the test was modeled.
A combination of 6 types (16, 18, 31, 33, 35, 45) resulted in 25% positive tests requiring treatment, with the potential to prevent up to 85% of all cervical cancers.
You can read the full text here; https://www.mdpi.com/2072-6694/15/22/5453
“The 6-type HPV mRNA test exhibited superior performance, and the suggested “test-and-treat” approach demonstrated substantial promise as a strategy for cervical cancer prevention in resource-constrained settings, with the potential to revolutionize current practices in cervical cancer prevention”
26 July 2023:
We are happy to share this work by Sørbye et al., reporting the prevalence and distribution of HPV types in 179 cervical cancer cases during a 20-year period preceding systematic HPV vaccination in Norway.
Understanding the distribution of HPV types in cervical cancer cases is crucial for evaluating the effectiveness of HPV screening and vaccination in reducing the burden of cervical cancer. Our findings demonstrated that 92.7% of the women diagnosed with cervical cancer between 1995 and 2015 in Troms and Finnmark counties tested positive for HPV in at least one test, identifying HPV 16 and 18 as the predominant types responsible for 70.8% of the cases.
Based on the genotype distribution reported from the pre-vaccine era, it was evident that the implementation of the bivalent or quadrivalent HPV vaccines could potentially prevent 76.4% of the HPV-positive cervical cancer cases, while the nonavalent HPV vaccine has the potential to improve prevention to 93.3%.
You can read the full text here: https://www.mdpi.com/2673-5261/4/3/15
8 June 2023:
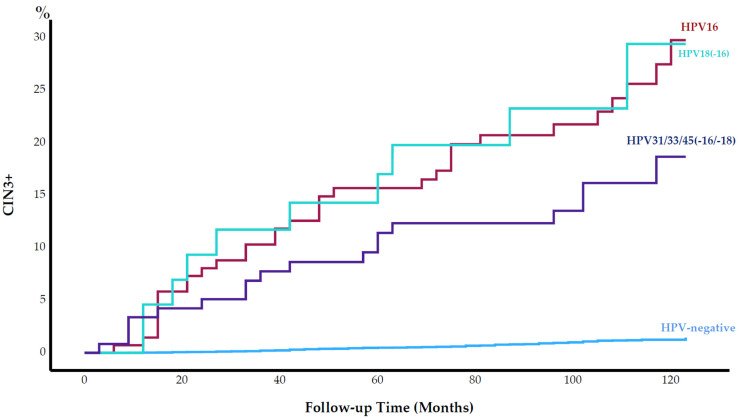
We are pleased to share this article by Rad et al., reporting the long-term performance of a five-type HPV mRNA test to predict cervical intraepithelial neoplasia grade 3 or worse (CIN3+) during up to 12 years of follow-up of 9582 cytology negative women at baseline.
The 10-year cumulative incidence of CIN3+ among the HPV mRNA-positive women was 20.8% (CI: 16.2–25.4), whilst 1.1% in HPV mRNA negative women at baseline.
The low long-term risk of CIN3+ among HPV mRNA-negative women, and the high long-term risk among HPV mRNA-positive women strengthens the evidence that the
five-type HPV mRNA test is an appropriate screening test for women of all ages.
You can read the full text here: https://www.mdpi.com/2072-6694/15/12/3106
Figure 1. Cumulative incidence of cervical intraepithelial neoplasia grade 3 or worse (CIN3+) (%) by human papillomavirus (HPV) type from baseline throughout 120 months of follow-up.
25 March 2023:
PreTect HPV-Proofer`7
-
a better way to manage HPV-infected women.
We are excited to share this important work by Sørbye et al. “Performance of a 7-Type HPV mRNA Test in Triage of HPV DNA Primary Screen Positive Women Compared to Liquid-Based Cytology”
A new study, nested in the real HPV-DNA primary screening in North Norway, presents evidence that a 7-type HPV mRNA test is a more precise risk stratification tool among HPV-infected women compared to liquid based cytology (LBC).
Evidence was presented that mRNA detection from the seven HPV types (16, 18, 31, 33, 45, 52, 58) represented a significantly higher risk of CIN2+ (29.0%) compared to LBC.
Presented data confirmed the 7-type HPV mRNA test to be significantly more specific than cervical cytology (71.0% versus 47.8%), reducing the number of colposcopies while keeping the same sensitivity towards the detection of CIN2+.
Using this biomarker as the threshold for colposcopy may better balance the benefits and harms of screening.
You can read the full text here: https://www.mdpi.com/2673-5261/4/2/8
22 November 2022:
Norwegian study emphasises the importance of HPV mRNA testing in young women- Impact of HPV mRNA types 16, 18, 45 detection on the risk of CIN3+ in young women with normal cervical cytology.
We are excited to share the new article by Al-Shibli et al.
The presented data for the 3-type HPV mRNA test, PreTect SEE, demonstrates that the expression of HPV mRNA 16, 18 and 45 represent a significant risk of future cervical abnormalities needing to be treated, even if the cytology is negative at the time of infection.
Screening young women with a 3-type HPV mRNA test can help avoid cervical cancers that may be missed when screening with cytology alone, while minimizing over referrals as opposed to screening by a 14-type HPV-DNA test.
Find out more information about how PreTect can help you to improve your cervical cancer screening here.
08 October 2020:
New Danish publication presenting the benefits of PreTect HPV-Proofer in the management of ASC-US/LSIL in young women.
Adopting primary HPV screening, young women continue to be screened by cytology, among whom the findings of ASC-US/LSIL are common. To obtain the best possible balance between reducing the risk of cervical cancer and limiting over-management, the choice of management strategy becomes essential.
The cohort study recently published by St-Martin et al, investigated how different management strategies used in Denmark influenced biopsy rates and detection of cervical intra-epithelial neoplasia (CIN) by comparing 5 main strategies: repeat cytology, 14-type mRNA test, 5-type mRNA test, DNA test or direct referral to colposcopy.
See excerpt from the text below.
“Main findings: The strategy used had an impact on the benefits and the harms of cervical screening of these young women. The detection of CIN3+ was somewhat higher, 13% to 14% with HPV testing vs. 11% with repeat cytology. Compared with repeat cytology, HPV testing led to more women undergoing biopsy, and more women had biopsies that turned out normal or with CIN1-2 which does not indicate treatment.
A specific triage test with a low positivity rate translates into a low referral rate for colposcopy, which is very appealing for triage situations. In our material, the 5-type HPV mRNA test was the most specific triage test.
Except for 5-type mRNA test, the relative risks for biopsies with < CIN2 were higher than the relative risks for high grade lesions, indicating over-management. The risk of having biopsies not indicating treatment was approximately doubled with 14-type mRNA test (2.11) or HPV DNA test (1.86) and almost tripled with direct referral to colposcopy (2.80).
In conclusion, the optimal strategy for assessment of ASCUS and LSIL is still not clear, and the choice will depend on the health authorities’ and women’s preferred balance between benefits and harms. Based on our study, repeat cytology or triage with 5-type mRNA test seems to offer advantages compared to HPV testing in terms of fewer unnecessary biopsies”.
Contact us to discuss how we can support you with risk stratification and actionable HPV mRNA genotyping to enhance patient management.
Further scientific publications regarding our products can be found on our Publications page.
Querétaro,
Invited speakers:
Prof. Finn Egil Skjeldestad
MD,PhD Sveinung Wergeland Sørbye
- Presenting the relevance and importance of HPV mRNA E6/E7 biomarkers in the prevention of cervical cancer
Årsmøte Den Norske Patologforening, Trondheim 7-8 mars 2019:
Årsmøte DNP Trondheim
“Overlege Sveinung Sørbye fra UNN holdt et engasjert innlegg om screening og forebygging av livmorhalskreft hos kvinner yngre enn 40 år og anbefalte rescreening av negative celleprøver med positiv 3-typers HPV mRNA test i denne gruppen”
Az Jan Palfijn, Gent
Zaterdag 16 februari om 9 uur
Lezing: Humaan Papilloma Virus (HPV)
en baarmoederhalskanker (taal : Engels)
Samenwerking tussen de dienst Anatomopathologie van AZ Jan Palfijn Gent en PreTect
iDx/Karyo,
Greece
HPV Symposium December 2018
Thessaloniki / Athens
The Excelsior / Αιθ. Mezzanine ακολουθεί δείπνο
Crown Plaza Athens City Center
DECEMBER 2018: EUROGIN, LISBON
PreTect HPV mRNA clinical data were presented in five free communications!
Because prevention is possible!
SPAIN - MEXICO - USA - NORWAY
May 2018:
Proudly presenting the new concept fighting cervical cancer in Spain:
Self-sampling by XytoTest - HPV Hr-DNA by Abbott and risk stratification by PreTect HPV-Proofer.
An unique combination!
"Mía by XytoTest®, a preventive comprehensive diagnostic system has completed its integration in Spain to prevent cervical cancer.
Our preventive protocol offers a unique sample collection technique ensuring sufficient amount and quality of cervical/vaginal specimens for HPV- testing. These samples will be analyzed targeting DNA to identify HPV-Hr infections using Abbott Laboratories technology.
In addition, to those positive samples for any HPV-Hr infection, a triage process will be implemented by detecting HPV mRNA E6/E7. The triage using the same original sample will risk stratify among the infections and indicate the ones most likely to progress to severe cervical disease or cervical cancer. By identifying the few women at highest risk, follow-up and treatment can be immediately effectuated, hereby preventing cervical cancer. The triage test is the most advanced mRNA method developed, patented by PreTect AS, Norway," said Mel-Mont Medical's COO, Frank Meléndez.